Surgeons and other procedural physicians ascribe, as do all physicians, to Primum non nocere, “First do no harm.”
The scope of the potential harm is realized when one considers that approximately 53 million outpatient and 46 million inpatient procedures are performed annually in the United States, including 18-20 million endoscopies.1,2,3
Because these involve contact with sterile tissue or mucous membranes, disinfection and sterilization are necessary to ensure that reusable instruments do not transmit disease to patients.2
Whether intervention is for diagnostic, palliative, or curative intent, inadvertent transmission of microbial or organic material is unacceptable in the potential risk posed to patients undergoing procedures.
However, relevant stewardship is not the exclusive domain of clinicians. Both the assured cleanliness and functionality of reusable surgical and other instruments are critical to providing appropriate care.
Therefore, an effective approach to patient safety and adverse events must be comprehensive and specifically inclusive of the role played by sterile processing departments.4 Such departments are often disconnected from proximity to the Operating or Procedure Rooms and are often located in basements.
There is often variability in process and among personnel, and there are a bevy of manufacturer-specific cleaning guidelines with concern for the sensitivity of particular devices and their surfaces to methods employed.1 The result is ongoing risk related to technical and human factors.
Leveraging 3M science for sterilization assurance
3M offers a broad portfolio of tools to facilitate patient safety through accurate, reliable testing as part of consistent and standardized workflows, and mitigation of human error in reusable equipment processing.
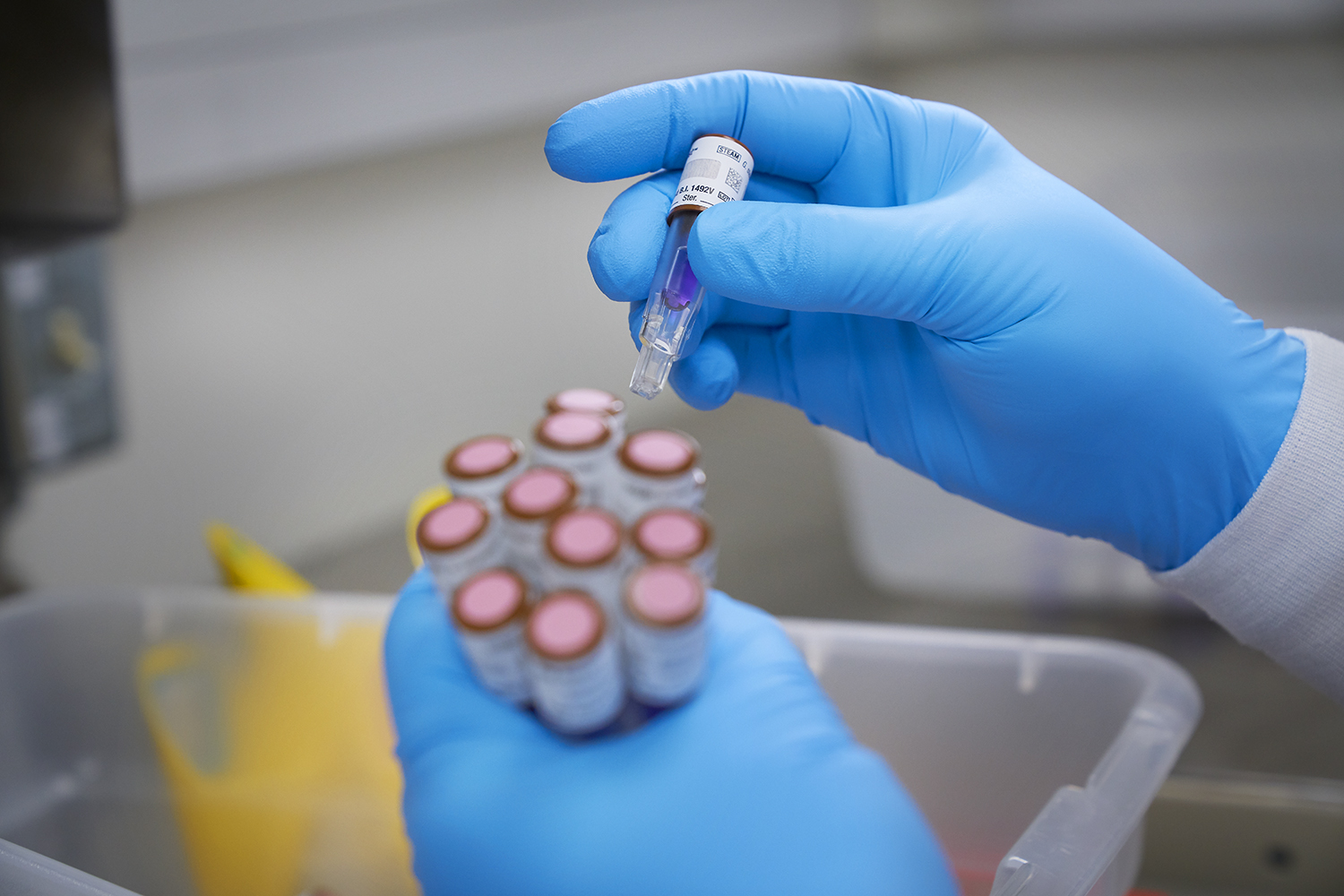
The monitoring of every sterilized equipment load affords the same level of protection to every patient; this process is enhanced by the speed of such indicators which allow the loads to be quarantined until satisfactory results are obtained (Figures 1 & 2).

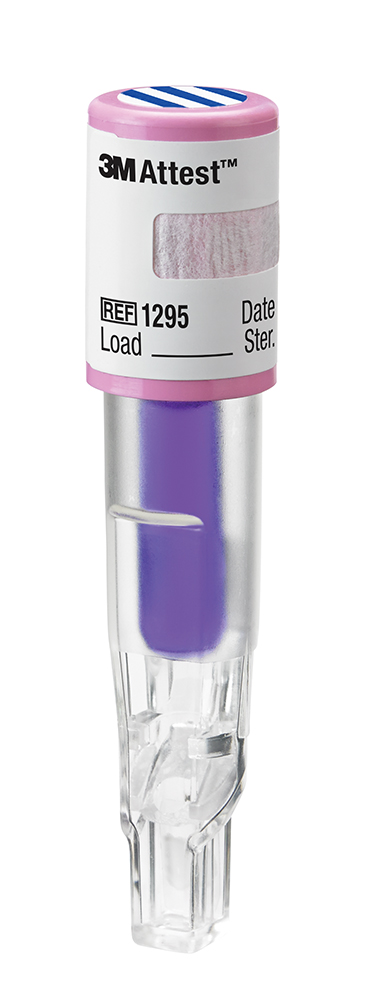

Figure 1: 3M™ Attest™ Biological Indicators for load monitoring using steam, vaporized hydrogen peroxide, and ethylene oxide sterilization.

![]()

Figure 2: 3M™ Comply™ Chemical Indicators for load monitoring using steam, vaporized hydrogen peroxide, and ethylene oxide sterilization.
3M scientists invented pressure-sensitive tapes more than 80 years ago. That adhesive expertise created chemical indicator tapes with reliable adhesion and color-change chemical indicator stripes that indicate exposure to steam sterilization or hydrogen peroxide sterilization processes. These exposure monitoring indicator tapes provide clear evidence that packs have been processed (Figure 3).


Figure 3: 3M™ Attest™ Indicator Tapes for load monitoring using steam and vaporized hydrogen peroxide.
Adenosine triphosphate (ATP) monitoring systems are valuable in monitoring cleaning processes for the full spectrum from reusable instruments and endoscopes to cleaned surfaces (Figure 4).
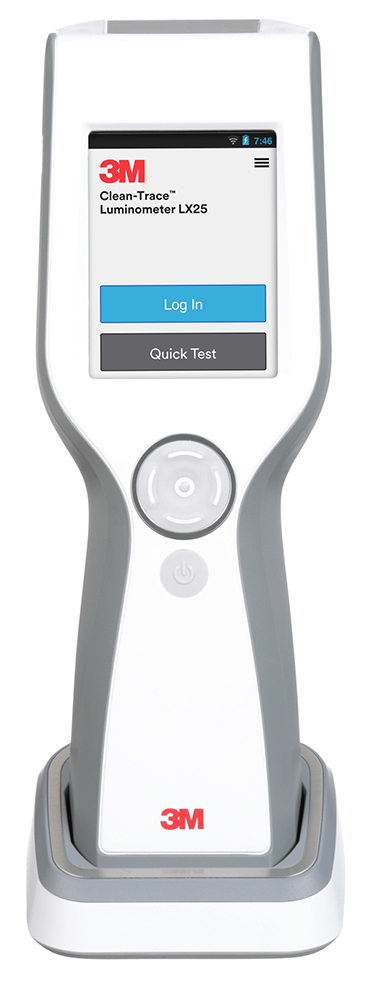

Figure 4: 3M™ Clean-Trace™ ATP Monitoring System, including the 3M™ Clean-Trace™ Luminometer LX25, the 3M™ Clean-Trace™ ATP Surface Test UXC, the 3M™ Clean-Trace™ ATP Water Test H2O, and the 3M™ Quality Control Data Manager (QCDM) online hosted service.
The 3M sterilization and cleaning monitoring portfolio also includes 3M™ Steri-Vac™ Sterilizer/Aerators and related equipment, 3M™ EO Abators, and supporting accessories for complete coverage (Figure 5).


Figure 5: 3M™ Steri-Vac™ Sterilizer / Aerator and 3M™ EO Abator.
Patient safety requires comprehensive and formalized processes and procedures that achieve appropriate cleaning, disinfection, and sterilization of reusable instruments and scopes. Rapid confirmation testing with each stage of the process and at completion provides both process optimization and contributes to mitigation of potential multifactorial breakdowns in system performance.
References:
1 Rutala WA and Weber DJ. Disinfection, sterilization, and antisepsis: An overview. Am J of Infection Control. 2019: 47: A3-A9.
2 Rutala WA and Weber DJ. Disinfection, sterilization, and antisepsis: Principles, practices, current issues, new research, and new technologies. Am J Infection Control. 2019; 47: A1-A2.
3 Alfa MJ. Biofilms on instruments and environmental surfaces: Do they interfere with instrument reprocessing and surface disinfection? Review of the literature. AJIC.2019; 47: A39-A45.
4 Brooks JV et al. The work of sterile processing departments: An exploratory study using qualitative interviews and a quantitative process database. Am J Infection Control. 2019; 47: 816-21.
