To say there is a lot of information pertaining to PPE and COVID-19 would be a vast understatement. We know this information can be complex, confusing, and difficult to know where to find the answers to the top questions. Decontamination of filtering facepiece respirators (FFRs), such as N95 respirators, has also become a significant topic of interest with the media, researchers and healthcare workers due to respirator shortages around the globe.
3M recently released information pertaining to the effect of certain decontamination methods proposed by sterilization companies and various healthcare institutions, on fit and filtration performance of 3M N95 respirators. This post breaks down this information and highlights important considerations to understand before choosing to decontaminate FFRs.
Optimizing the Supply of N95 Respirators
Helping to protect healthcare workers is of utmost importance and is a challenge we fully recognize. We also know that the current reality of product availability remains a prevalent issue that we are actively working to address. 3M is working to increase output of N95 respirators and is helping to get products to those who need it most, healthcare workers. That said, we also know you cannot wait. You need a solution now.
It’s important to consider the strategies or options to optimize supplies of disposable N95 filtering facepiece respirators when there is limited supply. However, understanding the strategies facilities have likely already put into place BEFORE we get to the point of discussing PPE is helpful to gain the larger picture of how we help reduce healthcare professionals’ exposure to airborne particles, to help mitigate infectious disease transmission.
Controlling exposures to occupational hazards is a fundamental way to help protect personnel. Conventionally, a hierarchy has been used to prioritize control efforts. Multiple control strategies can be implemented concurrently or sequentially. The Hierarchy of Controls shown here is from the U.S. CDC website Strategies for Optimizing the Supply of Facemasks:
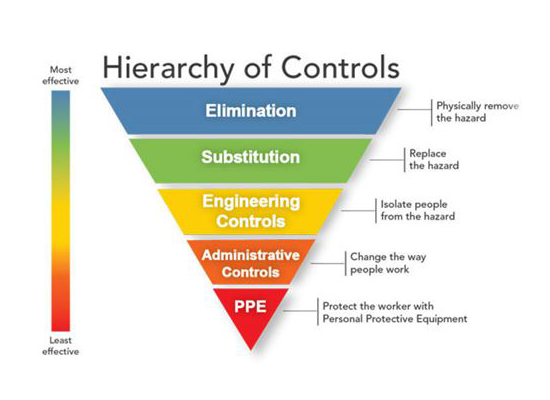
The most effective way of reducing the risk of disease transmission is elimination (physically removing the hazard) and then substitution (replacing the hazard). However, these are not typically options for healthcare settings. Exposures to transmissible respiratory pathogens in healthcare facilities can often be reduced through engineering and administrative controls and then, as the next preferred option, through implementation of PPE. This may come in the form of prompt detection and effective triage of patients or isolation of potentially infectious persons. Applying a combination of controls can provide an additional degree of protection, which may be beneficial if one intervention fails or is not available.
Surge capacity refers to the ability to manage a sudden, unexpected increase in patient volume that would otherwise severely challenge or exceed the present capacity of a facility. Under the CDC surge capacity framework, measures can be prioritized to conserve N95 respirator supplies along the continuum of care.
N95 respirators are the PPE most often used to help protect against exposure to airborne particulates, though their effectiveness is highly dependent upon proper fit and use. The optimal way to reduce the risk of airborne transmission is to use a combination of interventions from across the hierarchy of controls, not just PPE alone.
But what happens when there is a challenge to the supply of PPE?
Let’s explore the strategies that the CDC recommends prior to considering decontamination of FFRs:
1. Conventional capacity strategies
- Deploy the hierarchy of controls we just discussed, such as barrier implementation and telemedicine.
- Utilize existing supply with small changes in daily practices, such as conservation of supply and reevaluating use scenarios. Investigate ways to minimize the number of healthcare workers interacting with infected patients and the number of interactions with each patient.Contingency capacity strategies
2. Contingency capacity strategies
- Assumes the above has already been implemented and exhausted.
- May include measures such as suspending annual fit testing or extended use of N95s (using for multiple patient interactions).
3. Crisis capacity strategies
- May include use of respirators from other countries which are non-NIOSH approved, use of N95s past their shelf life, or limited reuse.
- Organizations practicing reuse of N95s should use a “bag and wait 5 days” approach to reusing if feasible and only decontaminate N95s as a last resort. Decontamination and subsequent reuse of N95 respirators should only be practiced as a crisis capacity strategy.
So, what are the recommendations for reuse of N95 respirators? A key excerpt from the CDC guidance on Decontamination and Reuse of Filtering Facepiece Respirators states in part:
- The healthcare worker will wear one respirator each day and store it in a breathable paper bag at the end of each shift.
- The order of FFR use should be repeated with a minimum of five days between each FFR use. This will result in each worker requiring a minimum of five FFRs, providing that they put on, take off, care for them, and store them properly each day.
- Healthcare workers should treat the FFRs as though they are still contaminated and follow the precautions outlined by the CDC.
If supplies are even more constrained and five respirators are not available for each worker who needs them, utilizing the capacity framework we’ve discussed, FFR decontamination may be necessary.
Decontamination Methods Explored
If your facility finds themselves deploying crisis capacity strategies, you can know we have been collaborating with several sterilization companies and institutions that are investigating ways for hospitals to safely decontaminate 3M N95 FFRs in line with the CDC guidance on Crisis Standards of Care Decontamination Recommendations.
It is important to understand our evaluation of these methods are in the context of their impact on fit and filtration performance and not their efficacy with regards to inactivation of the virus that causes COVID-19.
3M evaluates the effect of the sterilization method on filtration efficiency and integrity of our respiratory protection products. A respirator can only work when air passes through the filter. Air will take the path of least resistance, so if the seal isn’t there, the air will go around rather than through the respirator – and therefore lessen the protection.
If, as a result of decontaminating a respirator, the filtration is damaged or the respirator does not fit, it will not help reduce exposure to airborne particles at the level indicated, such as N95, FFP2, etc.
3M is in the process of testing certain 3M N95 FFRs treated by multiple sterilization companies and institutions regarding the effect of the decontamination processes on fit and filtration performance.
Through 4/29/2020, five companies’ commercial decontamination methods and systems—select STERIS and Advanced Sterilization Products (ASP) Vaporized Hydrogen Peroxide Sterilizer Systems — passed both our fit and filtration tests for certain 3M N95 models evaluated and have also received EUAs from the FDA to use their systems to decontaminate respirators as a crisis strategy during the pandemic. For more details and up-to-date information, see the 3M Technical Bulletin Decontamination Methods for 3M Filtering Facepiece Respirators such as N95 Respirators.
